How we Work
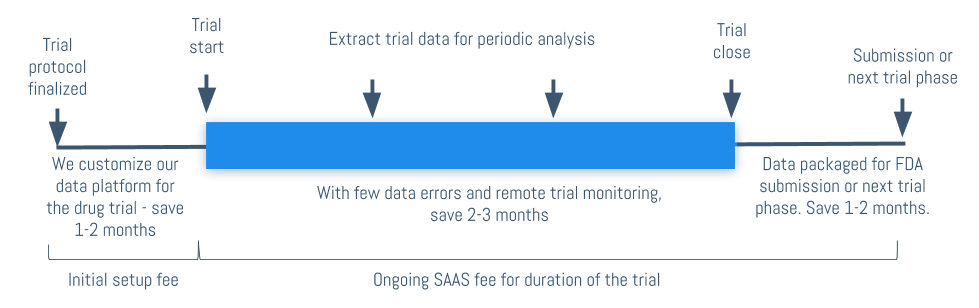
We build a data collection mobile app for your drug trial (mobile EDC), this will include data collection eCRFs, complete audit trail, data entry checks (edit specifications) and complete back and forth data querying functionality.
Once the study begins, you (or the CROs) can start cleaning the trial data and monitoring the drug trial.
Real-time mobile prescriptive/predictive analytics is fed back to the trial app. This includes up to the minute clinical and operational and clinical metrics for your trial.
Once the trial is done, you can download the entire database and write up the final study reports, go to the next trial phase or do a FDA/EMA submission.
Once the study begins, you (or the CROs) can start cleaning the trial data and monitoring the drug trial.
Real-time mobile prescriptive/predictive analytics is fed back to the trial app. This includes up to the minute clinical and operational and clinical metrics for your trial.
Once the trial is done, you can download the entire database and write up the final study reports, go to the next trial phase or do a FDA/EMA submission.

Pricing the trial is simple. We can provide a simple quote for the entire clinical trial for a shorter study (1-4 months/< 100 subjects) or for longer studies, pricing will involve initial setup costs and software as a service fee for the duration of the study.
